Molecules That Do Not Readily Dissolve in Water Are Called
Water does not associate with non polar molecules so the molecules maintain a tight membrane with the surrounding atmosphere when subjected to polar environments such as water. Water readily dissolves most polar compounds- true.

Water Water Is A Polar Molecule Composed Of Two Polar Covalent O H Bonds In A Bent Or Angular Molecular Geometry With Two Pairs Of Nonbonding Electrons Ppt Video Online Download
Substances which dissolve easily and readily in water sugar salt etc are called water-loving or hydrophilic substances.
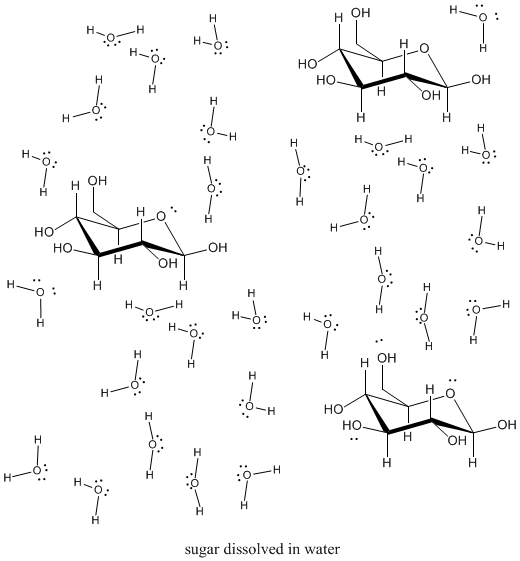
. 3The tendency of water to minimize its contact with nonpolar substances is called the hydrophobic effect. Oils fats and certain organic solvents do. Because water solubility depends on polarity nonpolar molecules do not readily dissolve in water.
Polar solutes such as sugars are ____ than the intermolecular attractions between solute molecules allowing solute molecules to readily dissolve in water. In other words hydrophobic molecules are nonpolar. Sugar sodium chloride and hydrophilic proteins are all substances that dissolve in water.
Hydrophobic molecules do not readily dissolve in water in part because water molecules involved in hydration cannot participate in normal hydrogen bonding with one another. Hydrophobic molecules do not readily dissolve in water in part because water molecules involved in hydration cannot participate in normal hydrogen bonding with one another. Therefore these molecules repel water molecules.
What molecules do not dissolve in water. Water interacts with polar solutes via its dipoles. Since many biomolecules are either polar or charged water readily dissolves these hydrophilic compounds.
Molecules that do not readily dissolve in water are called. Molecules with prevailing nonpolar bonds are the ones that are mostly insoluble in water and are called hydrophobic compounds. This is because water is hydrophilic.
Hydrophobic molecules do not dissolve in water. Dehydration synthesis and hydrolysis. Hydrocarbons containing C-H bonds are examples of hydrophobic compounds.
Hydrophobic molecules are individually hydrated in water increasing the entropy of the system. So the partially negative part of one polar molecule like water will interact with the partially positive part of another molecule like your mystery substance. Asked Jul 27 2018 in Anatomy Physiology by Janessa.
Table salt is not a molecular compound. Therefore pure water will not conduct electricity. 1Hydrophobic molecules do not readily dissolve in water in part because water molecules involved in hydration cannot participate in normal hydrogen bonding with one another.
This allows polar substances to dissolve each other. Another way to say this is that lipids which. Sugar sodium chloride and hydrophilic proteins are all substances that dissolve in water.
The solvent is the substance which typically determines the physical state of the solution solid liquid or gas. Hydrophobic molecules are molecules that do not dissolve in water. Substances which do not dissolve readily in water are called water-fearing or hydrophobic substances.
On the other hand some solutes are non-polar and do not have any positive or negative charges. If these forces are stronger than the ionic bond keeping the compound together it dissolves. There is a term known as hydrophobic or water-averse.
Many substances do not dissolve in water and that is because they are non-polar and do not interact well with water molecules. The water changes its hydrogen bonding patterns around the hydrophobic molecules to produce a cage-like structure called a clathrate. 84Molecules that do not dissolve well in water are called 84 A polar.
Can water be a solute. Water is capable of dissolving a variety of different substances which is why it is such a good solvent. Hence water is a polar solvent.
This is because water is hydrophilic. Can anything dissolve in water. Unlike polar molecules nonpolar molecules are hard to dissolve in water as they cannot react with carbon molecules.
Oils fats and certain organic solvents do not dissolve in water because they are hydrophobic. These hydrophobic molecules are called hydrophobes. These partial charges attract any opposite-charged ions or repel any similar-charged ions when an ionic compound in submerged in water.
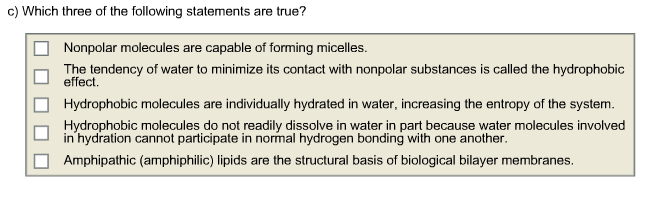
Hydrophobic molecules are hydrophobic due to their non-polarity. Nonpolar molecules which do not readily dissolve in water are called hydrophobic or water-fearing The Role of Water in Chemical Reactions Two types of chemical reactions involve the creation or the consumption of water. 2Nonpolar molecules are capable of forming micelles.
D The tendency of water to minimize its contact with nonpolar substances is called the hydrophobic effect. Water is a poor solvent however for hydrophobic molecules such as lipids. In an ionic compound one or more atoms loses electrons and one or more atoms gains electrons-true.
Hydrophobic molecules do not dissolve in water. And water is called the universal solvent because it dissolves more substances than any other liquid. Nonpolar molecules are capable of forming micelles.
This is because the intensity with which water molecules are attracted to C-H bonds is far lesser than the intensity towards other water molecules. It is an ionic compound. The hydrophobicity describes how much hydrophobic a molecule is.
A common example is oil and water. Oil contains molecules that are non-polar thus they do not dissolve in water. Nonpolar molecules experience hydrophobic interactions in water.
Table salt NaCL is a molecular compound- false. One part of the molecule will be more negative called partially negative and another part will be more positive called partially positive.
Solved Which Three Of The Following Statements Are True Chegg Com

Solved Which Three Of The Following Statements Are True Chegg Com

Which Three Of The Following Statements Are True Chegg Com

Ch 15 Solutions Water Is A Molecule And Therefore Water Forms Which Strongly Bonds The Molecules Together Ppt Download

Can Liquids Dissolve In Water Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
What Kind Of Compound Would Be Least Likely To Dissolve In Water Socratic
13 3 Solutions Of Solids Dissolved In Water How To Make Rock Candy Chemistry Libretexts

Solved Which Three Of The Following Statements Are True The Chegg Com

Solved Which Three Of The Statements Are True Hydrophobic Chegg Com

Do Polar Molecules Dissolve In Water Why Or Why Not Quora

Which Solids Dissolve In Water Cool Science For Kids

Properties Of Water Ppt Download
What Types Of Substances Dissolve Easily In Water Socratic

Natural Lipids Are Readily Soluble In Oil Mercury Water None Of

What Are Molecules That Do Not Readily Dissolve In Water Quora

Comments
Post a Comment